DW Documentary
“Big Pharma – How much power do drug companies have?” is a 42-minute documentary produced by DW Documentary and directed by Valentin Thurn. The film explores the pharmaceutical industry’s influence on health policy and the impact of its practices on public health systems.
The documentary highlights how some pharmaceutical companies develop highly profitable drugs with public money, while others have been found to have covered up serious side effects. The film also examines the role of large corporations, known as Big Pharma, in the pharmaceutical industry. These corporations are richer and more powerful than ever, and in some cases, they can even call the shots on governmental health policies.
The documentary features patients, whistleblowers, lawyers, doctors, politicians, and representatives of the industry. It accuses large laboratories of concealing or downplaying research results to maintain their monopolies. The film also discusses the lobbying efforts of manufacturer Gilead, as it seeks approval for a promising drug developed largely with public money.
Overall, “Big Pharma – How much power do drug companies have?” is a thought-provoking documentary that raises important questions about the pharmaceutical industry’s practices and their impact on public health.
* * *
The pharmaceutical companies hiking the prices of drugs. Martin Shkreli is the former CEO of Turing Pharmaceuticals, who’s responsible for hiking the price of at least one drug by five thousand percent.
‘Are you gonna change the price?’
‘No.’
Martin Shkreli is a phenomenon. He illustrates that the system is broken, and that the drug ecosystem is completely money-driven.
Richer and more powerful than ever, underpinned by its influence networks, the pharmaceutical industry stands unchallenged in its ability to dictate government health policies.
The industry’s power is comparable to that of a state.
The pharmaceutical industry is so rich and so powerful. Its lobbying affects Congress greatly and also the FDA.
While the industry benefits hugely from publicly funded research, it manages to steer healthcare expenditure towards its most expensive medications.
No good idea goes forward without a pharmaceutical company partner. And yet the current pricing is absolutely indefensible, in my opinion.
The pharmaceutical industry’s main concern is now profit. Their concern is shareholders, not patients.
It’s a very cruel business model because if you can’t pay for it, you don’t get it.
The battle against the COVID-19 pandemic has further wet the pharma company’s appetite. Has the pharmaceutical industry’s thirst for profit become a threat to our public health systems?
Daraprim is on the World Health Organization’s model list of essential medicines. An invaluable weapon in the fight against malaria and a serious infection caused by HIV. A young U.S. financier, Martin Shkreli, manager of a hedge fund investing in health products, bought the rights to the drug in the United States. He then bumped up the price of Daraprim from $13.50 to $750—a 5,000% increase.
Martin Shkreli refused to budge on the price of his medication.
Please welcome to the stage Martin Shkreli, founder…
Facing the financial press, he tried to defend the indefensible.
‘Thanks for coming.’
‘Thank you.’
‘Up here, if you could rewind the clock a few months, I wonder if you would do anything differently.’ ‘I probably would have raised the price higher, is probably what I would have done.
Why?
I think healthcare prices are inelastic. I could have raised it higher and made more profits for our shareholders, which is my primary duty. And again, no one wants to say it, no one’s proud of it, but you know, this is a capitalist society, capitalist system, and capitalist rules, and my investors expect me to maximize profits, not to minimize them or go half or go 70%, but to go to 100%.’
An immoral increase, maybe, but perfectly legal. As part of an investigation into the scandal of unchecked drug prices, Martin Shkreli was summoned to appear before a U.S. congressional committee.
‘What do you say to that signal pregnant woman who might have AIDS, no income, she needs Daraprim in order to survive. What do you say to her?’
‘On the advice of counsel, I invoke my Fifth Amendment privilege against self-incrimination and respectfully decline to answer your question.’
Somebody’s paying; it’s the taxpayers that end up paying for some of them. I know you’re smiling, but I’m very serious, sir.
I ask now that the committee excuse the witness from the table. Without objection, so ordered. We’ll pause for a moment as Mr. Shkreli is escorted out.
Martin Shkreli was soon dubbed the most hated man in America. Some time later, he was arrested by the FBI and convicted by a federal court on securities fraud. He was sentenced to seven years in federal prison. Beyond his individual case, Shkreli became the embodiment of the excesses of the pharmaceutical industry and the embodiment of its cynicism. The price of Daraprim has never returned to its original level.
Some useful fools attract attention by their behavior, which, while cynical, is actually very useful in giving a clear picture of what these people and their abuses of the system represent.
Over the past 10 years, the landscape of the drug industry has dramatically changed. A handful of pharmaceutical companies manufacture the vast majority of drugs. One of the world’s top five is Swiss company Novartis, having acquired a host of smaller companies. It boasts an annual turnover of 45 billion dollars and now holds some promising patents and treatments for cancer and other rare diseases. The two American giants Pfizer and Johnson & Johnson have also bought dozens of rival firms to expand their markets. Another top five company is Roche, a Swiss drug manufacturer which has acquired 25 competing therapies, and the French giant Sanofi has an annual turnover of more than 40 billion dollars. It too has bought a dozen pharmaceutical laboratories in Europe and North America. With each having 100,000 employees, these multinationals make up the global market commonly known as ‘Big Pharma.’
To maintain their monopoly on certain diseases, large laboratories are accused of playing down or hiding from the health authorities some of the results of their clinical trials. The upshot is that some of the drugs released on the market trigger serious side effects in the patients they are supposed to be treating. Like Mediator, a drug produced by Servier Laboratories from France and withdrawn from the market 30 years after launch amid a major scandal. Or Depakine, an epilepsy treatment, one of the world’s most widely distributed drugs in the last 50 years, made by the French giant Sanofi. The drug, while effective, is at the center of a huge health scandal in Europe. Its use has been proved to be extremely dangerous for the unborn child in pregnant women.
I often compare my condition to the effects of a violent electric shock, like a short circuit in an electric box. Everything disconnects. I lose consciousness, suffer convulsions, then come round a few minutes or a few hours later. The fear of never waking up is a constant. So for me, medication is vital. No question about it. Without medication, I wouldn’t be here today.
I was well prepared for my pregnancy. I knew I had to consult my doctor first. I asked my family physician, ‘Is this suitable for pregnant women?’ I was told it was fine. My gynecologist and neurologist told me the same thing.
Nathan was born with a urogenital abnormality. Time went on, and Nathan passed the age when he should have started talking and sitting up. He didn’t either.
Marine Martin and her husband became increasingly worried. They filmed their son Nathan to document his delayed development as evidence to doctors. An abnormally calm child, he did not smile, lacked muscle tone, and exhibited signs of language delay and relationship disorders.
And I was told he wasn’t a late developer. It was a disorder. Nathan suffers from severe neurological sequelae. Marine Martin embarked on an extensive investigation into the side effects of the drug Depakine, eventually becoming a whistleblower and filing a complaint against Sanofi in a bid to have the company’s responsibility recognized. She’s created an advocacy group with thousands of victims. In France and Switzerland, several individual lawsuits and a class action are currently underway against Sanofi. The firm is under investigation for aggravated deceit and accidental injuries.
It was also a way for me to make amends. The guilt was overwhelming. When you take a medication every day, twice a day in my case, and poison your baby. It’s still difficult for me now. So for me, the process of making amends and exposing Sanofi’s deceit was essential. I couldn’t have left with myself. I had to help the other families.
Depakine. The victims’ grievance is that they were not forewarned about the risks of taking the drug during pregnancy.
According to the health authorities, since the drug was first marketed in 1967, tens of thousands of children in Europe have suffered birth defects and mental impairment.
What you have here is a pharmaceutical laboratory that doesn’t play the information game and fails to communicate all the information it has to the health authorities. It may not have been 100% sure, but we know that the lab wrote in 2003 that it had been aware of the issues since 1970. The laboratory’s reply is, “Yes, but the risk seemed slight.”
Sanofi is one of the main Big Pharma companies, the biggest in France in terms of research and development of new drugs, with branches in 100 countries worldwide. Their slogan is “Empowering Life.” After weeks of negotiations, their management agreed to an interview under one condition: Sanofi refused to discuss the ongoing legal proceedings in the cases of Marine Martin and victims’ associations in Europe.
How long have those in charge at Sanofi been aware of Depakine’s serious side effects?
To answer your question, first, Depakine is used in the treatment of epilepsy, that’s the therapy for which we were licensed to sell the product. Remember, epilepsy is a serious condition. Sanofi has been rigorous in monitoring and supplying information to the health authorities, medical profession, and patients.
The health authorities say your application lacked detail and your warnings were poorly explained.
I don’t think we are vague; we report all the information we have in our possession. The Inspector General of Social Affairs carried out a thorough examination of the Depakine case. It concluded in 2016 that it was scientifically impossible, given the data available at the time, to conclude a direct correlation between valproate and neurodevelopment, prior to 2004. We approached the health authorities in 2003.
Despite everything, how do you explain your failure to convince the health authorities to act faster in informing medical professionals and, above all, patients who might be pregnant?
It is the responsibility of our company to ensure, at all times, that the information we gather about the use of this product, pharmacovigilance, scientific developments, be constantly transmitted to the health authorities. That’s our duty, and we do that regularly, systematically, and transparently.
While the evidence was piling up on the link between the drug Depakine and serious disorders affecting children, it took another 11 years for patients to be alerted. In 2015, Sanofi finally came to an agreement with the ANSM, the official body that licenses drugs in France. The patient package insert supplied with Depakine was amended to clearly indicate the significant risk of deformity and developmental problems. Almost 50 years after the drug was first launched, despite all of the warnings.
The warning in the patient package insert was finally included in 2015, in extremely precise terms. Would you mind reading the first hard-hitting paragraph?
No, no.
You’d rather I did it?
Yes, go ahead.
Sanofi’s patient package insert is now very clear in all languages. Depakine can seriously harm an unborn baby when taken during pregnancy. Exposed children are at high risk of serious intellectual and motor development disorders in up to 30 to 40 percent of cases, and of deformities in around 10 percent of cases.
Their argument is the state licensed me to sell this product, so don’t blame me. But wait, in the Volkswagen dieselgate scandal, the government deemed the cars roadworthy, but it is Volkswagen’s responsibility to pay for the lies and the defects in their cars, not the government’s. It’s absurd! Manufacturers are responsible for their products; with Depakine the authorities were remiss, but the main responsibility lies with the manufacturer.
Only after a two-year battle was Marine Martin able to ensure a warning would be affixed to the Depakine label, like those found on alcoholic drinks.
All pharmaceutical companies are looking for what they call a “blockbuster,” a drug that treats widespread diseases and can be marketed worldwide. To preserve exclusive rights, Big Pharma companies have developed some powerful strategies.
This is the story of a revolutionary treatment for AMD (age-related macular degeneration), a severely disabling eye disease that can lead to blindness. Millions of patients are affected worldwide. It’s also the story of the competition between two equally effective drugs, one costing 40 times more than the other. Until 2005, there was no effective treatment, and many patients lost their sight.
Then came a new drug that significantly slows the progression of the disease, a liquid injected directly into the eye. The therapy had been developed in the United States and was said to revolutionize treatment of the disease. Several major ophthalmologists launched clinical trials on thousands of patients they were unable to treat.
It’s the leading cause of permanent blindness in people over the age of 65. In fact, perhaps for any disease worldwide
There is a role for pharmaceutical companies, particularly in research and development of new medications. They invest many years and a lot of dollars. That being said, they are for-profit companies, and their objective is to find what they call the “blockbuster drug” that they can sell a lot of to a large population. Which is why there is a lot of R&D (research and development) going into macular degeneration because it is such a common disorder.
This first treatment was developed by the American laboratory Genentech. It was called Avastin, newly licensed by the FDA in America. The drug was officially used to treat colon cancer. Then, by accident, an American professor discovered that its properties significantly delay the development of AMD and improve eyesight.
Progress is slow, and it causes legal blindness.
Phil Rosenfeld began experimenting with intravenous Avastin for the treatment of neovascular AMD.
There were really no studies done by the pharmaceutical company to do that. It was really some doctors and a pharmacist who worked together to come up with what might be the optimal dosing of Avastin in the eye.
The treatment worked flawlessly.
Professor Rosenfeld presented his results at the annual ophthalmology conference.
The results were like nothing we’d ever seen before. Every retina specialist that I know who was at that meeting, our jaws were on the floor. Because for the first time, we had seen an improvement in visual acuity, whereas every treatment that came before it had a decline in vision over a one-year period. We’d never seen that before.
And because Avastin was priced to be given for cancer, it was inexpensive.
So for the first six to twelve months, everyone all over the world was using Avastin to treat these patients until Lucentis came out.
Meanwhile, ophthalmology researchers at the very same American Genentech pharmaceutical laboratory that manufactured Avastin for cancer were developing a new treatment specifically for AMD: Lucentis
Introducing Lucentis, a breakthrough in neovascular AMD. Lucentis redefines efficacy…
All the studies showed that the two treatments were identical, except the new Lucentis treatment was much, much more expensive.
For Avastin, that would make Avastin about fifty dollars an injection, and then when Lucentis comes out, a single dose in the eye is two thousand dollars.
Meanwhile, in France, pharmacists in hospitals were becoming interested in treating the eye condition with the Avastin used for cancer.
Avastin is dosed in bottles for injection into the eye. Pharmacists had to repackage it in syringes at a lower dose.
You take a file, a small bottle containing 16 milliliters, then use it to fill a number of syringes, maybe a batch of 50 for ophthalmological injections. It entails repackaging the contents of a bottle into an injectable form. The really important thing is to ensure the preparation is completely sterile before delivering it for administration by the ophthalmologist. The syringe must be absolutely sterile. This meant we could make syringes for around 50 euros each. You have to include staff costs and testing costs, plus the premises and equipment have to be paid for. So the cost for us was 20 times less than it was for Lucentis, which cost around a thousand euros a syringe.
In Europe, while expensive, the cost of the new Lucentis eye treatment was still only half what it was in the United States, where prices are not controlled. The drug was marketed by Swiss laboratory Novartis. Avastin is sold by the other big Swiss laboratory, Roche, which bought out the American company Genentech.
Novartis and Roche – Especially Roche – don’t like it when products they sell to treat a specific condition are used by us to treat other medical conditions, used for purposes other than those designated.
Novartis and Roche then concocted a strategy to prevent doctors from using Avastin to treat eye conditions and convince them to inject Lucentis.
Were you visited by representatives of Novartis or Roche at that time?
About that treatment? Absolutely, yes. They came to say they didn’t understand why we were using Avastin, a cancer treatment, to treat ophthalmological conditions.
I had a meeting with the general manager of Novartis who came to the hospital to ask me why I insisted on making these Avastin syringes when Lucentis was a licensed treatment. So I explained my thinking, which was how best to serve the public and patients, allied to economic and health considerations. The conversation was mostly about the potential danger to patients.
Did you think Novartis was putting pressure on you?
If it was pressure, it carried no threats.
Since all international studies demonstrated the equal effectiveness of the two treatments, hospital pharmacists naturally favored the least expensive option. The two laboratories, Roche and Novartis, launched a long legal procedure against the French state. The maneuver was doomed to fail. Avastin was eventually authorized for use in France, but it was too late.
The problem now for the health system is that it has a setup that is so complex for ophthalmologists to manage that basically everyone has given up.
Thus, almost all patients treated for AMD were given an injection of the more expensive products, including the one made by Novartis.
In Italy, in 2014, Roche and Novartis were fined some 180 million euros for illicit price-fixing of the two drugs. In late 2020, France fined Roche and Novartis 444 million euros.
There were people in the academic community who really liked using Lucentis. It’s a great drug. I have the utmost respect for the role that the pharmaceutical industry plays in our field. No good idea goes forward without a pharmaceutical company partner. And yet the pricing, to me, particularly when you’ve got a fifty-dollar disruptor that’s equally effective, the current pricing is absolutely indefensible, in my opinion. It’s estimated that the US saves three billion dollars a year by using Avastin instead of Lucentis.
According to the regulations, the French Medicines Agency cannot force Roche to manufacture Avastin syringes for the treatment of AMD. Despite two months of negotiations, the multinational Roche refused to be interviewed. Its legal department told us, ‘We do not wish to speak or be filmed on this subject but are more than willing to answer any questions you may have in writing.’
Question: Why does the laboratory refuse to manufacture Avastin syringes for the treatment of AMD?
Answer: Roche develops drugs only for medical needs not covered by existing drugs, where there are no therapeutic alternatives.
Roche then says no, and the public authorities are powerless to force their hand. Lobbying has paid off; the cheaper alternative is rarely used. The big winner is Novartis, which coincidentally owns a third of Roche.
In the United States, the price of drugs is completely unregulated. As soon as the FDA approves the drug, the pharmaceutical companies are free to impose their prices. Elected officials, Democrats and Republicans alike, persist in trying to force manufacturers to lower the cost of new treatments, in vain. One recent medication has changed the health economy: an overpriced treatment that has launched a new scramble for profit between the biggest pharmaceutical companies.
This is a major American discovery: a new treatment against hepatitis C. It helps cure the often deadly chronic liver disease by eradicating the virus. Sovaldi, made by Gilead, the world’s 10th largest pharmaceutical company, was launched on the U.S. market in 2014. The price of the three-month treatment: $84,000. That’s a thousand dollars a tablet.
Sovaldi is the first of the drugs that actually cures hepatitis C in three months. The virus is gone. It is manufactured by what is now a very big drug company called Gilead Sciences. But Gilead had nothing to do with the research that discovered Sovaldi. Gilead Sciences bought Pharmasset, and they bought it because that way they could get their hands on Sovaldi. They are concentrating more on diseases that don’t affect that many people. Better life and death diseases, so they can charge whatever they want. So they charged eighty-four thousand dollars. The sales of Gilead Sciences came to 32.5 billion dollars, of which 55 percent, this is according to the annual reports, was pure profit.
The American drug then hit the European market at half the price. The price of treatment for a course of Sovaldi was 42,000 euros. The still exorbitant cost aroused a great deal of outrage among patients. The NGO Medecins du Monde launched an awareness campaign in France. 230,000 patients were affected. At 42,000 euros a pop, in terms of health insurance, the hepatitis C treatment was one of the most expensive around.
We can thank Gilead for marketing Sovaldi because they showed the wider public that today drugs are not sold for the price they should be sold at. It reflects the necessity for Gilead to recover the huge costs involved, having spent 10 billion. They had to fix a high price.
I heard that hepatitis C could be treated. The treatment now available was expensive, but it cured the disease in 12 weeks. Patients who had, for the most part, until then been condemned by hepatitis C. Gilead was in a position of relative strength, having a unique treatment that offered a cure. Negotiations about that were rather robust and tense. Then got underway. The price today has come down by more than half.
Under pressure, the American pharmaceutical lab lowered the price of the three-month Sovaldi treatment in France from 42,000 to 24,000 euros. A U.S. Senate commission revealed the company’s confidential marketing strategy.
We had access to thousands of pages of internal Gilead documents. So we could see that in Gilead meetings, marketing ideas were aired. In particular, ideas for pricing, but no mention was ever made of the actual outlay for R&D, manufacture, and marketing.
In this internal presentation, the Gilead sales team recommends an introductory price of between eighty thousand and eighty-five thousand dollars, dismissing concerns about any potential scandal in the press.
The sole concern was how to maximize profits, achieve strong financial margins, cover costs, then move on. Public health considerations were never an issue.
Marisol Touraine now director of Unitaid, a body operating within the World Health Organization charged with negotiating significant price reductions with large pharma companies to treat patients in the southern hemisphere. Seventy-one million people worldwide carry the hepatitis C virus.
The drop in price was considerable and was established by introducing generic drugs and giving the pharma lab assurances that we would open major markets rather than sell a little at a high price we offered the chance to sell a lot at a lower price. We scored a victory by ensuring treatment was accessible at a reasonable price.
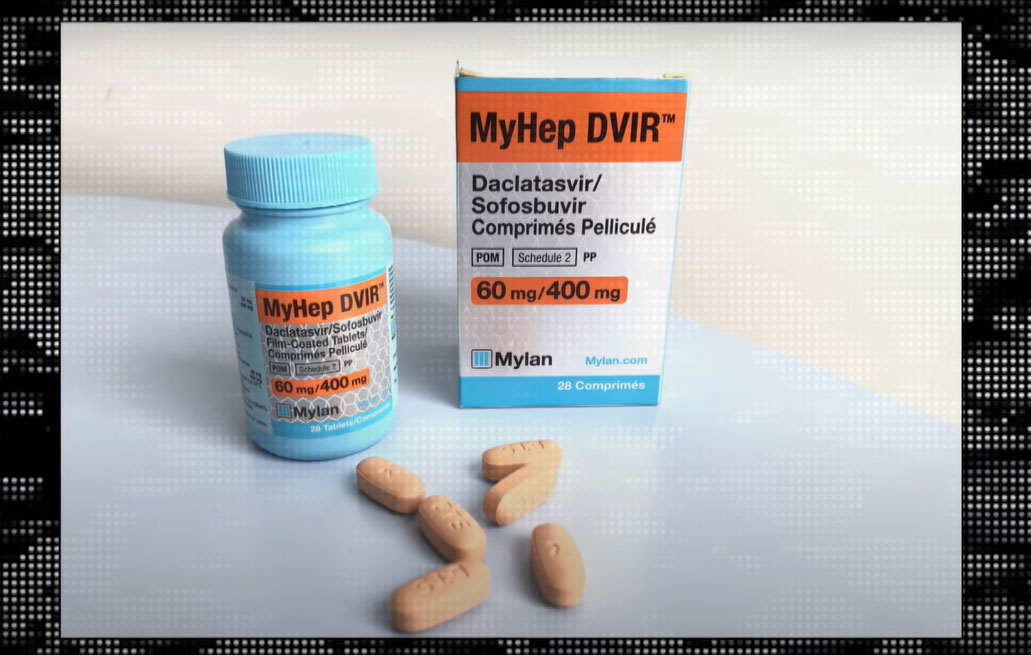
Thus, treatment for hepatitis C using the generic drug produced by Mylan costs less than eighty dollars, available only in developing countries. In the northern hemisphere, a course of treatment still costs tens of thousands of euros.
The price of drugs no longer reflects the real cost of research, rather the financial power of a few large companies scrambling for a disproportionate profit. With the appearance of new gene therapies to treat certain cancers or rare diseases, prices are still increasing, reaching several hundreds of thousands of euros. All the major big pharma companies are in the race for these new treatments. They save lives, but at what cost?
A new gene therapy to fight cancer is now marketed by Swiss company Novartis to treat leukemia. Its name is Kymriah. Its price is 320,000 euros per patient.
The therapy was discovered by a team of publicly funded university researchers at the University of Pennsylvania. But flexing its financial muscles, Novartis became co-owner of the patent.
Professor of hematology Jean-Paul Vernant is a renowned expert in blood cancers.
There’s a whole technique involved using gene therapy. A gene is introduced, which allows T cells to target diseased cells and enable them to be destroyed. It’s a very interesting system, but there’s no justification for charging 350,000 euros. If state structures in France went along with it, it would doubtless cost 30 or 40,000, but not 350,000 euros.
Obviously, it’s the pharmaceutical industry’s job to keep a lookout for something interesting. The research is no longer carried out by the pharmaceutical industry. The work is tackled by publicly funded research.
The drug companies, they don’t do their own innovation anymore. They do zero innovation or close to zero. The innovation still comes from NIH-funded research. What we’re seeing now is a big change that I think is terrible. And this big change is, instead of being focused almost exclusively on the size of the market, on volume, it’s now focused on price. They acquire a drug that’s effective against a serious disease, multiple sclerosis, cancer, serious disease that people will pay almost anything to get, and jack up the price so that it can cost hundreds of thousands of dollars for a year’s treatment, I mean hundreds and thousands of dollars. It’s a very cruel business model because if you can’t pay for it, you don’t get it.
Cancer treatments now cost something like 300,000 or 400,000 euros, while the number of cancer patients in France is around a million eight hundred thousand with four hundred thousand new cancer cases every year. I cannot see how in the future we will be able to treat more than 1.5 million people when the price scale is some 300,000 euros.
There’s a whole sales pitch relating to the therapeutic value and life-saving value of these medications, which has been skillfully put forward by opinion leaders to convince governments, which basically says that drugs heal. They are a unique product. The high cost is the price of life. It’s pure marketing. Better cures mean higher prices.
The problem is that each country defends its multinationals. France defends Sanofi, the USA defends Pfizer and the big American companies. Switzerland defends Novartis and Roche. It’s hard to reach consensus to bring down prices to reasonable levels.
After two months of negotiations, the multinational Novartis turned down our request for an interview. Instead, they sent a simple press release: ‘We price our new medicines based on the value they deliver to patients, healthcare systems, and society. We strive to take into account income levels, local affordability barriers, and economic realities while maintaining the sustainability of our business.’ Novartis tells us, as its representatives have explained to political decision-makers, that this new single-dose cancer therapy must be compared to a treatment of drugs taken for life. In late 2019, the laboratory agreed to a slight decrease in France, where the price of Kymriah went down from 320,000 euros to 297,666 euros.
The FDA now is on the payroll of the pharmaceutical industry. They pay user fees to the part of the FDA that evaluates new drugs for approval. So this makes this part of the FDA dependent on the companies that they are supposedly regulating. The drug companies love it because it makes the part of the FDA that evaluates their drugs extremely friendly. Since they support it, it is a blatant conflict of interest. This ought to be well-funded, and there ought to be no conflicts of interest.